| Diagnostic imaging | |
FDA approves pre-market application for Siemens mammography system
23 August 2004
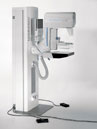
MALVERN, Pa. The U.S. Food and Drug Administration (FDA) has granted approval of the Pre-Market Approval Application (PMA) to Siemens Medical Solutions for the MAMMOMATNovationDR a full-field digital mammography system. The new MAMMOMAT NovationDR meets the demands of modern mammography practices by providing digital screening, diagnosis, and stereotactic biopsy capabilities — all in one system. “The MAMMOMAT NovationDR offers a comprehensive solution for modern mammography that optimizes workflow, and furthers Siemens’ ability to offer one of the most comprehensive women’s health solutions available today. With this innovative new system, screening, diagnosis and biopsy all can be performed on a single unit,” said Holger Schmidt, president of the Siemens Medical Solutions Special Systems Division. “Thanks to the combination of proprietary detector technology and our unique X-ray tube, both image quality and dose exposure reduction achieve an optimum level. In addition, the system’s acquisition and reporting stations deliver optimized workflow. Clearly, Siemens is offering an investment in the future of digital mammography.” The new MAMMOMAT NovationDR features an innovative flat panel detector based on amorphous Selenium (aSe) detector technology. This technology enables a direct conversion of X-ray to digital information, unlike some other detector systems, such as amorphous silicon (aSi-CsI), which use indirect x-ray conversion. The aSe detector technology has the potential to provide higher spatial resolution and greater clinical detail. At 24 by 29 centimeters, the image detector size of the MAMMOMAT NovationDR also enables imaging of a larger range of patient sizes, and the system features a new paddle designed for easier and more comfortable patient positioning. A unique feature of MAMMOMAT NovationDR is the MammoReportPlus, our dedicated workstation for mammography, which provides high-volume mammogram reading and optimized workflow. The system’s dedicated keypad and roaming/panning function gives easy-to-full spatial resolution and allows users to switch between eight-view mammographic studies in less than one second. MammoReportPlus also is designed to meet future imaging needs and is prepared for digital computer-aided (CAD) applications. The MAMMOMAT NovationDR digital system is based on the company’s flagship analog mammography product—the MAMMOMAT 3000 Nova. The connectivity features of both the MAMMOMAT 3000 Nova and the new MAMMOMAT NovationDR are clear examples of Siemens’ demonstrated leadership in image data and workflow management, and will facilitate the new system’s integration into a digital network, potentially improving patient management and care. More information can be obtained by visiting: www.usa.siemens.com |