| Business, neurology | |
EU approval and first European implant for ANS neurostimulation system19 May 2006 Plano Texas, USA. Advanced Neuromodulation Systems (ANS), the neuromodulation business of St. Jude Medical, Inc. (NYSE:STJ), has received European CE Mark clearance for its Eon rechargeable neurostimulation system, and announced the first European patient implant.
Neurosurgeon Athanasios Koulousakis, M.D., performed the first European implant at University Hospital in Cologne, Germany. “The Eon was easy to implant and the patient has responded well to the targeted pain relief this neurostimulation therapy offers,” said Dr. Koulousakis. “Eon is a good option for patients who need high power stimulation settings and want the convenience of a rechargeable device.” The Eon implantable pulse generator (IPG) is designed to last a minimum of seven years at high power settings. This allows patients to go longer between battery replacement surgeries. Eon can power up to 16 independent electrodes, which allows clinicians more programming options to better manage the patient’s pain. “Physicians throughout the European Union will now have a broader choice among neurostimulation systems to treat their patients,” said Chris Chavez, president of ANS. “We believe that patients will appreciate how easy the system is to use. They simply recharge the device periodically, just as they would their cell phone.” Spinal cord stimulators like Eon are implanted devices that are similar in function and appearance to cardiac pacemakers. To have a spinal cord stimulator or “pain pacemaker” implanted, a patient must undergo a minor surgical procedure in which a lead or leads are placed in the epidural space and connected to a generator, which serves as the power source and programming “brain.” Once activated, the system’s programs are adjusted and fine tuned to best control the patient’s pain. Patients use a controller (similar to a remote control) that allows them to check the system’s battery, adjust the power level, select from pre-set programs and turn the system power on and off. Chronic pain is a largely under treated and misunderstood disease that affects millions of patients worldwide. It is defined as pain that persists for more than six months after an injury, reoccurs periodically over six months, and continues for an indefinite period of time. As many as one in five people suffers from moderate to severe chronic pain, and one in three people is unable or less able to maintain an independent lifestyle due to pain (according to the World Health Organization in conjunction with The European Federation of the International Association for the Study of Pain). The Eon System was approved by the U.S. Food and Drug Administration (FDA) in 2005. More than 25,000 patients in more than 25 countries around the world use ANS neurostimulation therapies to manage chronic pain.
|
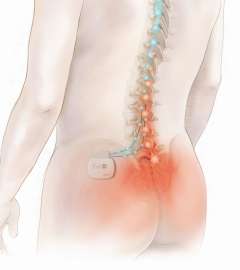 The
Eon Neurostimulation System helps patients manage chronic, intractable pain
of the trunk and limbs, including pain associated with failed back-surgery
syndrome, by using low-intensity electrical impulses to selectively trigger
nerve fibres along the spinal cord. It is though that stimulating these
nerve fibres diminishes or blocks the intensity of the pain message being
transmitted to the brain, replacing feelings of pain with tingling
sensations.
The
Eon Neurostimulation System helps patients manage chronic, intractable pain
of the trunk and limbs, including pain associated with failed back-surgery
syndrome, by using low-intensity electrical impulses to selectively trigger
nerve fibres along the spinal cord. It is though that stimulating these
nerve fibres diminishes or blocks the intensity of the pain message being
transmitted to the brain, replacing feelings of pain with tingling
sensations.