Graphene-based nanoelectronics a step closer
31 January 2009
The mass production of graphene-based nanoelectronics has been brought a step closer with the discovery of a new method for controlling the nature of graphene by researchers at Rensselaer Polytechnic Institute in New York.
Graphene, a one-atom-thick sheet of carbon, was discovered in 2004 and is considered a potential heir to copper and silicon as the fundamental building blocks of nanoelectronics.
With help from an underlying substrate, researchers for the first time have demonstrated the ability to control the nature of graphene. Saroj Nayak, an associate professor in Rensselaer’s Department of Physics, Applied Physics, and Astronomy, along with Philip Shemella, a postdoctoral research associate in the same department, have determined that the chemistry of the surface on which graphene is deposited plays a key role in shaping the material’s conductive properties. The results are based on large-scale quantum mechanical simulations.
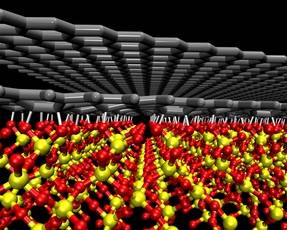 |
| A a rendering of two sheets of graphene, each with the thickness of just a single carbon atom, resting on top of a silicon dioxide substrate. |
Results show that when deposited on a surface treated with oxygen, graphene exhibits semiconductor properties. When deposited on a material treated with hydrogen, however, graphene exhibits metallic properties.
“Depending on the chemistry of the surface, we can control the nature of the graphene to be metallic or semiconductor,” Nayak said. “Essentially, we are ‘tuning’ the electrical properties of material to suit our needs.”
Conventionally, whenever a batch of graphene nanostructures is produced, some of the graphene is metallic, while the rest is semiconductor. It would be nearly impossible to separate the two on a large scale, Nayak said, yet realizing new graphene devices would require that they be comprised solely of metallic or semiconductor graphene. The new method for 'tuning' the nature of graphene is a key step to making this possible, he said.
Graphene’s excellent conductive properties make it attractive to researchers. Even at room temperature, electrons pass through the material effortlessly, near the speed of light and with little resistance. This means a graphene interconnect would likely stay much cooler than a copper interconnect of the same size. Cooler is better, as heat produced by interconnects can have negative effects on both a computer chip’s speed and performance.
Results of the study were published this week in the paper, Electronic structure and band-gap modulation of graphene via substrate surface chemistry, in the January 19 2009 issue of Applied Physics Letters.
Bookmark this page