OrSense receives EU approval for non-invasive haemoglobin and pulse oximetry monitoring system
8 April 2009
OrSense Ltd has received a European CE Mark for its NBM-200MP, a multi-parameter sensor for non-invasive continuous hemoglobin, low signal oximetry and pulse rate measurements.
"We are excited about receiving the European CE approval for NBM-200MP. This is an important milestone for OrSense in the path for developing and marketing our unique non-invasive multi-parameter blood monitoring device, which is expected to enhance the quality of treatment and reduce operational costs in hospitals," said Lior Ma'ayan, CEO of OrSense. “Upon receiving the CE mark, we will soon initiate sales in various counties in Europe.”
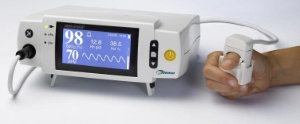
The Orsense NBM-200MP blood monitor
Hemoglobin (Hb) level, a parameter which indicates haemorrhage or anaemia, is a key measurement in hospitals and outpatient wards. However, current hemoglobin measurements are invasive, time consuming, labour intensive and costly.
Non-invasive pulse oximetry has become a standard of care throughout the medical world, yet it has been known to provide erroneous oxygen saturation readings in states of low perfusion, low cardiac output and/or low blood flow.
The NBM-200MP system offers for the first time a non-invasive, continuous and accurate measurement of oxygen saturation under all physiological conditions, as well as a unique, breakthrough non-invasive solution for accurate continuous and spot Hb measurements.
Bookmark this page