MicroPhage opens clinical trial on microphage-based platform to identify MRSA
1 September 2009
MicroPhage has launched a multi-site clinical trial to support a US FDA premarket notification [510(k)] for its bacterial identification platform based on bacteriophage amplification. The platform has been developed to rapidly identify bacterial infections and determine antibiotic susceptibility or resistance to aid physicians in antibiotic management.
The company’s first product is designed to rapidly identify Staphylococcus aureus (staph) bacteria and determine methicillin resistance (MRSA) or susceptibility (MSSA) in suspected cases of bacteremia — bacteria in the blood, in as little as 5 hours. Today’s standard of care for determining these types of infections takes up to three days.
The MicroPhage test platform requires no instrumentation and is composed of two small reaction tubes for incubating blood culture specimens. After five hours, the incubated samples are added to a dual dipstick-like detector, which looks much like a pair of home pregnancy tests. One part of the detector shows if the sample is infected with S. aureus bacteria and the other shows if it is susceptible or resistant to the antibiotic.
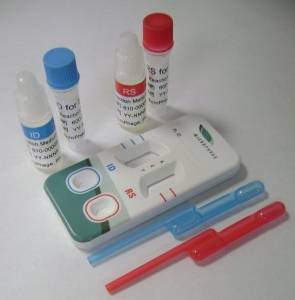
The MicroPhage S. aureus / MRSA / MSSA blood
culture test kit.
(Photo: Business Wire)
Results allow for more precise antibiotic therapy for a condition that has a mortality rate of 20% or more. Delivering this diagnostic information quickly will enable physicians to prescribe more effective and precise antibiotics that could shorten hospital stays, lower rising health care costs, and ultimately save lives.
The study will involve seven major medical centers throughout the US and is expected to test more than 2,000 specimens to demonstrate its safety and performance. The MicroPhage test will be compared to a laboratory 'gold standard' test to determine performance. It is expected to be completed in the fourth quarter of this year.
“This is a very exciting time for our company,” said MicroPhage CEO, Steve Lundy. “We are excited about our prospects to provide health care providers with rapid, actionable information to fight the rising tide of hospital acquired infections while lowering health care costs. We look forward to this Trial and getting to market later this year.”
About the MicroPhage technology
MicroPhage has adapted bacteriophage-amplification, a natural biologic process, for identifying bacterial infections. Bacteriophage are harmless bacteria-specific viruses that multiply aggressively when exposed to target bacteria.
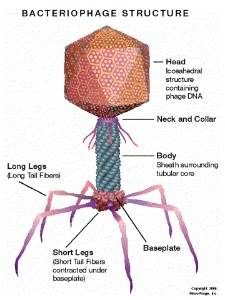
Major Parts of a Lytic Bacteriophage.
(Graphic: Business Wire)
In the detection process, reaction of the bacteriophage proteins on the test strip indicates the sample is positive for staph bacteria. For susceptibility analysis, the organism in the sample is challenged with an antibiotic.
Because bacteriophage depend on host bacteria for amplification, any compound that kills or inhibits the microbe will stop phage amplification. Only strains resistant to the antibiotic allow this amplification and yield a positive signal on the second detector strip, indicating an MRSA infection.
About Staph infections
Staphylococci are frequently implicated in bloodstream infections (BSI) with high morbidity and mortality. In a multinational study, 36% of bloodstream isolates were staphylococci, 61% of which were Staphylococcus aureus.
In a prospective cohort of patients with hospital-acquired BSIs in the United States, S. aureus was a primary cause, accounting for 20% of cases. The incidence of S. aureus bacteremia has increased significantly over the past decade, largely due to the increasing use of intravascular catheters and invasive devices.
There has also been a significant rise in rates of methicillin-resistant S. aureus (MRSA). Almost 60% of S. aureus bacteremias in the United States are now caused by these resistant strains. Despite advances in medical therapy and diagnostic procedures, S. aureus bacteremia is often associated with serious complications with a mortality rate that exceeds 20%, especially if appropriate therapy is not administered rapidly.
A rapid and reliable test for this diagnosis would allow clinicians to optimize diagnostic and therapeutic decisions. Antibiotic therapy could be adjusted early, leading to better health outcomes for patients with lower pharmacy and hospitalization costs.
Diekema DJ, Schmitz FJ, Pfaller MA, Bell J, Smayevsky J, Beach M, Jones RN, and the SENTRY Participants Group. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY antimicrobial surveillance program, 1997–1999. Clin Infect Dis 2001;32:S114–S132
Bookmark this page