Zilico to exhibit pioneering device for rapid detection of cervical cancer at Medica
3 November 2009
A pioneering device that offers a quicker, more accurate detection of cervical cancer in real time, removing several weeks of waiting for a diagnosis, is being exhibited by Sheffield-based Zilico Ltd for the first time at Medica 2009.
Zilico's APX is a portable hand-held device that measures the resistivity of cells and detects any changes as they progress from normal through to a cancerous state.
Cervical cancer affects around 500,000 women worldwide each year and is responsible for 300,000 deaths so the ability of the APX to provide objective and accurate results in ‘real-time’ means it will offer a quicker diagnosis for both patients and clinicians worldwide.
It will help to better manage the disease and also significantly reduce morbidity due to over-treatment and under-treatment due to false negative smears.
The system is safe and painless and has two functionalities within the one unit: the APX 100, which supports clinicians within their diagnostic procedure and the APX 200, which is a point-of-care (POC) test.
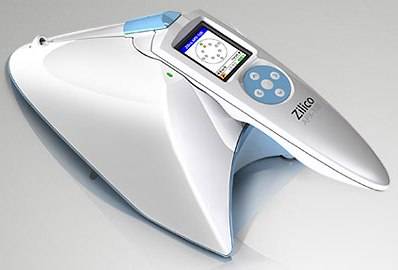
The Zilico APX
The APX100 enables colposcopists to better target biopsy sites helping to reduce the number of diagnostic biopsies and avoid the gross over-treatment of mild abnormalities. It has undergone four separate clinical trials and is currently in the middle of an EU multi-centre trial. Once this is completed, it will be launched across the European Union in 2010.
The APX 200 application is being targeted at the cervical screening market as it provides a real-time diagnosis at the primary point-of-care thus reducing the significant traffic in the healthcare system between primary care, cytology laboratories and colposcopy clinics. Ultimately it will result in a more effective application of limited healthcare resources.
Sameer Kothari, Chief Executive of Zilico Limited, said: “In countries where organised screening is still not established the APX 200 provides an opportunity to rapidly deploy screening programmes without the significant capital infrastructure costs of building and running pathology laboratories. It is safe, painless, and accurate and we are delighted to be presenting it at MEDICA 2009.”