Orteq reports knee improvement with Actifit meniscal tissue repair product
1 Oct 2010
Orteq Sports Medicine has reported that a two-year study has found significant pain reduction and functional improvement in patients' knees repaired with its meniscal scaffold product Actifit.
Actifit is a meniscal scaffold for the treatment of irreparable partial meniscal tears.
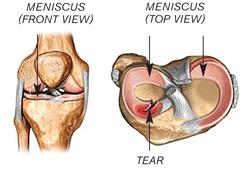
Meniscal tears are the most common injury of the knee, affecting at least 1.5 million people globally each year. In young patients, meniscal tears normally occur as a result of sports injuries, with the most common mechanism involving twisting on a loaded bent knee with the knee then giving way. The knee's menisci are two half-moon, wedge-shaped pieces of cartilage that act as a shock absorber distributing forces evenly between the femur (upper leg) and tibia (lower leg) in the knee joint.
Developed over the last two decades by leading polymer scientists and orthopaedic surgeons treating sports injuries, Actifit is designed to help patients regain an active lifestyle after suffering a meniscal injury.
The arthroscopically implanted polyurethane scaffold provides a temporary structure that supports the ingrowth of new tissue to replace the surgically removed damaged meniscal tissue. In time, Actifit degrades and is replaced by new functional tissue with meniscus-like characteristics.

Close-up of the honeycomb structure that
promotes new tissue growth
Professor Rene Verdonk, head of the Department of Orthopaedic Surgery and Traumatology at the University Hospital in Gent, Belgium, and lead investigator of the Actifit EU study group, gave his overall impression of the two year results:
“I am delighted with the two-year results. Young patients previously hindered by pain have returned to pre-injury activity levels and can now enjoy a normal life having been treated with Actifit. The product is safe and reliable, and we had a very low failure rate.
"It can be clearly seen securely in place on MRI at two years and the biopsies done of patients at one year showed there is consistent in-growth of vital new tissue in each biopsy. Actifit appears to be the next generation solution for the treatment of painful irreparable partial meniscus tissue loss.”
Dr Eva-Lisa Heinrichs, Chief Medical Officer of Orteq commented: “The most exciting data from the study is related to the patients’ decreased pain at the same time function has increased. After a period of rehabilitation, patients can gradually increase their activity level and return to pre-injury activities.
"In fact, statistically significant improvements were seen in all clinical scores used in the study (VAS, IKDC, KOOS, and Lysholm), with the biggest improvement observed in pain reduction and in the KOOS subscales which assess return to sports and quality of life.
“It has been great to work with the Actifit Study Group, as well as the specialists in the Orthopaedic, Radiology and Cell Biology departments at the University Hospital of Gent, Belgium. Their expertise has been invaluable in completing and analysing the Actifit EU clinical study data.”
Professor Hans Pässler of the ATOS Klinik in Heidelberg, Germany, a knee surgeon and key member of the study group, commented: “Having worked with numerous new technologies for addressing knee injuries, Actifit seems a most promising breakthrough. Importantly, I find it easy to use in the operating room and it works very effectively when used in the right patients.”
Dr Heinz Laprell, from the Lubinus Clinicum in Kiel, Germany and one of the most experienced users of Actifit, said: “My Actifit patients have significantly improved. I was very happy to be part of the Actifit study for this exciting next generation technology for symptomatic irreparable meniscus tears. I look forward to sharing my Actifit results at various congresses in the coming months and I will publish my results separately in addition to the Actifit study results.”
Orteq CEO Dianne Blanco said: “The Actifit 2 year results are outstanding. We have both excellent patient pain and efficacy scores. This robust clinical data underpins our belief that Actifit offers patients an important new option for the treatment of irreparable partial meniscal tears.”
Actifit has been launched across Europe through a network of independent distributors and is available in the following countries: Germany, United Kingdom, Italy, France, Spain, Portugal, Belgium, Netherlands, Switzerland, Austria, Norway, Sweden, Finland, Denmark and Greece.
What is a meniscus tear?
The knee joint is the most complex and remarkable joint in the body. It is capable of withstanding high load and shear stresses. Integral to the knee's functionality is the meniscus. The knee's menisci are two half-moon, wedge-shaped pieces of cartilage (the lateral and medial meniscus), acting as a shock absorber, lubricant and elastic buffer, distributing forces evenly between the femur (upper leg) and tibia (lower leg) in the knee joint.
At least two-thirds of the meniscus is both thin and avascular (has no blood supply). About 80% of meniscal damage occurs in the avascular area where successful long-term tissue repair is not possible.
Each year, orthopaedic/sports medicine surgeons treat some 1.5 million meniscal injuries in Europe and the United States alone. Many of these injuries are originally sports-related from golf, skiing, soccer or other active pursuits. A sudden twist of the knee can result in a pinch or tear of the meniscus. At present, if the tear is in the non-vascularised area, there is only one surgical option and the damaged tissue is removed by performing a partial meniscectomy. This leads to a permanent reduction in the functionality of the knee joint.
When injuries occur in the vascularized area of the meniscus (about 20% of the time), it is possible to repair the tear by suturing or using fixation devices. However, treatment fails in approximately 20% of these cases within two years of the initial surgery at which stage there is no option but to remove the injured tissue.
There is overwhelming clinical evidence that removal of meniscal tissue leads to a degenerative condition over time. This is followed by articular cartilage damage, often leading to painful osteoarthritis (bone and cartilage degeneration) and frequently results in a total knee replacement. There is a strong demand for products that will promote the regeneration of new meniscus tissue.