CRDM prototypes aortal sleeve for Marfan syndrome sufferers
1 March 2011
CRDM, a specialist in component prototyping, has partnered with Exstent to offer a groundbreaking solution for supporting the weak aorta of Marfan syndrome sufferers.
Marfan syndrome is an inherited disorder of the body’s structural tissues that affects 12,000 people in the UK. It causes a deficiency in fibrillin — a structural protein fibre — and can affect many areas of the body. The most detrimental consequence involves the aorta — the main arterial conduit from the heart, which runs the risk of dilating and ultimately rupturing.
A sufferer of Marfan syndrome himself and unhappy with the existing surgical options available, process engineer and Exstent founder Tal Golesworthy set out to find an alternative solution to major heart surgery — a daunting prospect for many sufferers of the disorder.
The question posed was, if the aorta is only weak in tensile strength within its wall; why not simply externally support it with a bespoke sleeve in a suitable material? The answer to this was the creation of the EARS (external aortic root support).
After combining magnetic resonance imaging (MRI), computer-aided design (CAD) and rapid prototyping (RP), Golesworthy put together a proposal to manufacture a bespoke external support for the ascending aorta.
Enlisting the help of reputable figures in the medical field and with the support of private investors, Tal started working on the EARS project. Existing medical MRI was used to get suitable images and Imperial College, London, was contracted to assist with the required CAD work. The next stage was to transform the imaging into rapid prototyping.
CRDM was chosen for converting the CAD stl files into solid polymeric formers for manufacture of the EARS implants.
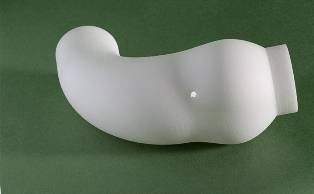
An aortal sleeve
As each implant needs to be unique to the individual patient, they must all be distinctively identifiable. CRDM has been actively involved in solving this problem. To ensure that each former is given to the correct patient, CRDM carries out an alphanumeric recessed text process situated on a non-morphologically sensitive part of the former. This gives surgeons the ability to distinguish one former from another.
After the manufacture of the former had been completed, it was taken to a cleanroom environment where it was solvent washed and dried before the textile EARS implant was formed onto it.
To date, 23 patients including Golesworthy have received the implants, all of which have been manufactured by CRDM. The manufacturing procedure will remain unchanged until the breakthrough surgery reaches 50 patients. After this milestone, the process will be reviewed, addressing everything from imaging protocol through to CAD routine, rapid prototyping and manufacturing methods.