First implantable wireless programmable microchip for controlling drug delivery
24 February 2012
MicroCHIPS, Inc. has published the results of the first successful human clinical trial with an implantable, wirelessly controlled and programmable microchip-based drug delivery device. Blood levels of an osteoporosis drug were similar to that from multiple injections.
The MicroCHIPS study was published in the online edition of the journal Science Translational Medicine.
"These data validate the microchip approach to multi-year drug delivery without the need for frequent injections, which can improve the management of many chronic diseases like osteoporosis where adherence to therapy is a significant problem," said study lead author Robert Farra, MicroCHIPS President and Chief Operating Officer.

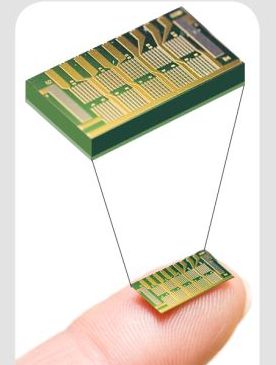
The MicroCHIPS implantable chip
"We look forward to making further progress to advance our first device toward regulatory approvals, as well as developing a range of products for use in important disease areas such as osteoporosis, cardiovascular disease, multiple sclerosis, cancer, and chronic pain."
In the trial, post menopausal women diagnosed with osteoporosis received daily doses of the marketed osteoporosis drug teriparatide through microchip delivery rather than daily injection. The drug released from the implanted microchip demonstrated similar measures of safety and therapeutic levels in blood to what is observed from standard, recommended multiple subcutaneous injections of teriparatide.
In the study, seven osteoporotic postmenopausal patients between the ages of 65 and 70 received the microchip-based implant. The primary objective of the clinical trial was to assess the pharmacokinetics (PK) of the released drug teriparatide from the implanted devices. Safety measures included evaluation of the biological response to the implant and monitoring indicators of toxicity. Secondary objectives were to assess the bioactivity of the drug and to evaluate the reliability and reproducibility of releasing the drug from the device.
The device and drug combination were found to be biocompatible with no adverse immune reaction. The resulting PK profiles from the implant were comparable to and had less variation than the PK profiles of multiple, recommended subcutaneous injections of teriparatide. The study also demonstrated that the programmable implant was able to deliver the drug at scheduled intervals. Drug delivery and evaluation in patients occurred over a one month period and provided proof-of-concept measures of drug release and device durability that support implantable device viability for 12 months or more.
The microchip device was implanted and explanted using local anaesthetic. Patient surveys found that the microchip device was well-tolerated, and patients indicated that they would repeat the implant procedure. "Each procedure lasted less than 30 minutes," said treating surgeon Pia Georg Jensen, MD. "The patients were able to walk out of the facility and go home unescorted."
To assess efficacy and improvement in bone fracture risk, the study measured biological markers of bone formation (P1NP), and bone resorption (CTX). In the study, changes in serum calcium, P1NP, and CTX resulting from drug implant therapy were found to be qualitatively and quantitatively similar to those reported in previous studies during daily subcutaneous injections of teriparatide.
"A microchip that continues to deliver teriparatide with this or similar consistency and efficiency over 12 to 24 months could improve bone mass, density, architecture, and strength," said study co-author Robert Neer, Founder & Director of the Massachusetts General Hospital Bone Density Center and Associate Professor of Medicine at Harvard Medical School.
Implantable medical devices such as pacemakers and pain pumps perform important functions to help patients return to a healthier state and to manage their disease. The design of a next-generation microchip drug delivery device is the only approach to an implantable device that can be wirelessly programmed to release drugs inside the body without percutaneous connections in or on the patient. An implantable microchip device also provides real-time dose schedule tracking, and as part of a network, physicians can remotely adjust treatment schedules as necessary.
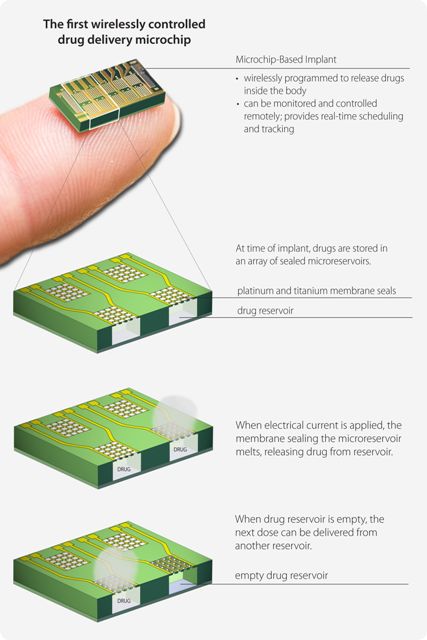
"This trial demonstrates how drug can be delivered through an implantable device that can be monitored and controlled remotely, providing new opportunities to improve treatment for patients and to realize the potential of telemedicine," said study co-author Robert Langer, ScD, Institute Professor at the David H. Koch Institute for Integrative Cancer Research at MIT, and cofounder of MicroCHIPS, Inc. "The convergence of drug delivery and electronic technologies gives physicians a real-time connection to their patient’s health, and patients are freed from the daily reminder, or burden, of disease by eliminating the need for regular injections."
MicroCHIPS is currently developing new designs of its microchip-based implant to include as many as 400 doses per device providing daily dosing for one year or multi-year therapy for less frequent dosing regimens. Components of the original microchip technology, such as the array of micro reservoirs used to contain drug and the first microchip opening mechanism, were developed at the Massachusetts Institute of Technology and licensed to MicroCHIPS.
The study with the implantable microchip in osteoporosis patients was funded and managed by MicroCHIPS. In addition to osteoporosis, the company is advancing applications for the microchip device for other therapeutic applications in proprietary programs and through strategic partnerships. The company plans to file for regulatory approval for its first microchip device in 2014.