First patient receives new implant that replaces middle ear
23 January 2012
A Swedish patient has become the first to receive an implant that replaces a malfunctioning middle ear and restores some hearing through 'bone conduction'. The unique invention from the Chalmers University of Technology was implanted as part of a clinical study. The operation was performed on the patient in December 2012.
With the new hearing implant, developed at Chalmers in collaboration with Sahlgrenska University Hospital in Gothenburg, Sweden, the patient has an operation to insert an implant slightly less than six centimetres long just behind the ear, under the skin and attached to the skull bone itself. The new technique uses the skull bone to transmit sound vibrations to the inner ear, so-called bone conduction.
The technique has been designed to treat mechanical hearing loss in individuals who have been affected by chronic inflammation of the outer or middle ear, or bone disease, or who have congenital malformations of the outer ear, auditory canal or middle ear.
Such people often have major problems with their hearing. Normal hearing aids, which compensate for neurological problems in the inner ear, rarely work for them. On the other hand, bone-anchored devices often provide a dramatic improvement. In addition, the new device may also help people with impaired inner ear.
“You hear 50% of your own voice through bone conduction, so you perceive this sound as quite natural”, said Professor Bo Håkansson, of the Department of Signals and Systems, Chalmers.
Unlike the type of bone-conduction device used today, the new hearing implant does not need to be anchored in the skull bone using a titanium screw through the skin. The patient has no need to fear losing the screw and there is no risk of skin infections arising around the fixing.
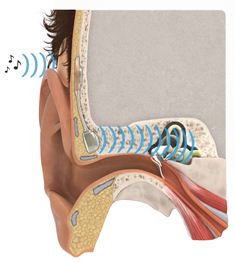
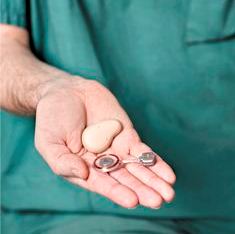
The device has two parts, one exterior processor and one implant The implant is slightly less than six centimetres long. By a surgical procedure, it is inserted just behind the ear, under the skin, into the bone itself. The coil at the upper end operates using magnetic induction with the outer, visible component, a sound processor that the patient easily can attach to or remove from the head.
The external sound processor is held in place using two magnets. The titanium screw through the skin, used in other techniques, is replaced by an inductive link that transmits sound from the patient’s surroundings through the intact skin to an internal receiver. The audio signal is transmitted to a tiny quadratic loudspeaker anchored to the bone near the auditory canal. The speaker generates sound vibrations which reach the sensory organs of the cochlea. The toughest challenge has been to make it sufficiently small, while effective enough.
“Once the implant was in place, we tested its function and everything seems to be working as intended so far. Now, the wound needs to heal for six weeks before we can turn the hearing sound processor on”, said Måns Eeg-Olofsson, Senior Physician at Sahlgrenska University Hospital, Gothenburg, who has been in charge of the medical aspects of the project for the past two years.
“Patients can probably have a neural impairment of down to 30-40 dB even in the cochlea. We are going to try to establish how much of an impairment can be tolerated through this clinical study”, said Bo Håkansson.
If the technique works, patients have even more to gain. Earlier tests indicate that the volume may be around 5 decibels higher and the quality of sound at high frequencies will be better with BCI than with previous bone-anchored techniques.
Now it’s soon time to activate the first patient’s implant, and adapt it to the patient’s hearing and wishes. Then hearing tests and checks will be performed roughly every three months until a year after the operation.
“At that point, we will end the process with a final X-ray examination and final hearing tests. If we get good early indications we will continue operating other patients during this spring already”, said Måns Eeg-Olofsson.
The researchers anticipate being able to present the first clinical results in early 2013. But when will the bone-conduction implant be ready for regular patients?
“According to our plans, it could happen within a year or two. For the new technique to quickly achieve widespread use, major investments are needed right now, at the development stage”, said Bo Håkansson.