Behaviour and function of enteroviruses monitored using gold nanoparticles
17 January 2014
Researchers at the Nanoscience Center (NSC) of University of Jyväskylä in Finland have developed a novel method to study enterovirus structures and their functions by attaching gold nanoparticles to the surface of viruses so they show up in imaging.
.The method will help to obtain new information on trafficking of viruses in cells and tissues as well as on the mechanisms of virus opening inside cells. This new information is important for example for developing new antiviral drugs and vaccines.
Enteroviruses are pathogenic viruses infecting humans. This group consists of polioviruses, coxsackieviruses, echoviruses and rhinoviruses. Enteroviruses are the most common causes of flu, but they also cause serious symptoms such as heart muscle infections and paralysis. Recently, enteroviruses have been linked with chronic diseases such as diabetes (2).
The infection mechanisms and infectious pathways of enteroviruses are still rather poorly known. Previous studies in the group of Dr. Varpu Marjomäki at the NSC have focused on the cellular factors that are important for the infection caused by selected enteroviruses (3). The mechanistic understanding of virus opening and the release of the viral genome in cellular structures for starting new virus production is still largely lacking. Furthermore, the knowledge of infectious processes in tissues is hampered by the lack of reliable tools for detecting virus infection.
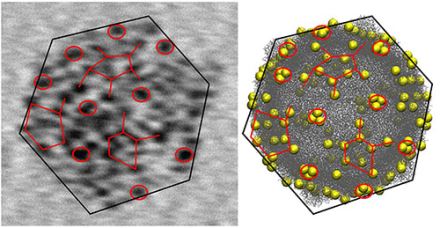
Left: transmission electron microscopy (TEM)
image of a single CVB3 virus showing tens of gold nanoparticles
attached to its surface. The particles form a distinct “tagging
pattern” that reflects the shape and the structure of the virus. The
TEM image can be correlated to the model of the virus (right), where
the yellow spheres mark the possible binding sites of the gold
particles. The diameter of the virus is about 35 nanometers (nanometer
= one billionth of a millimeter). The figure is taken from
publication (1).
The newly developed method involves a chemical modification of a known thiol-stabilized gold nanoparticle, the so-called Au102 cluster that was first synthesized and structurally solved by the group of Roger D Kornberg in 2007 (4) and later characterized at NSC by the groups of prof. Hannu Häkkinen and prof. Mika Pettersson in collaboration with Kornberg. (5)
The organic thiol surface of the Au102 particles is modified by attaching linker molecules that make a chemical bond to sulfur-containing cysteine residues that are part of the surface structure of the virus. Several tens of gold particles can bind to a single virus, and the binding pattern shows up as dark tags reflecting the overall shape and structure of the virus (see the figure). The gold particles allow for studies on the structural changes of the viruses during their lifespan.
The study showed also that the infectivity of the viruses is not compromised by the attached gold particles which indicates that the labeling method does not interfere with the normal biological functions of viruses inside cells. This facilitates new investigations on the virus structures from samples taken from inside cells during the various phases of the virus infection, and gives possibilities to obtain new information on the mechanisms of virus uncoating (opening and release of the genome). The new method allows also for tracking studies of virus pathways in tissues. This is important for further understanding of acute and chronic symptoms caused by viruses. Finally, the method is expected to be useful for developing of new antiviral vaccines that are based on virus-like particles.
References
(1) V. Marjomäki, T. Lahtinen, M. Martikainen, J. Koivisto, S.
Malola, K. Salorinne, M. Pettersson and H. Häkkinen, "Site-specific
targeting of enterovirus capsid by functionalized monodisperse gold
nanoclusters", Proc. Natl. Acad. Sci. USA (2014),
www.pnas.org/cgi/doi/10.1073/pnas.1310973111
(2) O. H.
Laitinen, H. Honkanen, O. Pakkanen, S. Oikarinen, M. M. Hankaniemi,
H. Huhtala, T. Ruokoranta, V. Lecouturier, P. Andre, R. Harju, S. M.
Virtanen, J. Lehtonen, J. W. Almond, T. Simell, O. Simell, J. Ilonen,
R. Veijola, M. Knip, H. Hyoty. “Coxsackievirus B1 Is Associated with
Induction of β-Cell Autoimmunity That Portends Type 1 Diabetes”,
Diabetes, 2013; DOI: 10.2337/db13-0619
(3)
www.jyu.fi/bioenv/en/divisions/smb/varpu
(4) P.D. Jadzinsky,
G. Calero, C.J. Ackerson, D.A. Bushnell and R.D. Kornberg,
“Structure of a thiol monolayer-protected gold nanoparticle at 1.1 Å
resolution“, Science 318, 5849 (2007).
(5) E. Hulkko, O.
Lopez-Acevedo, J. Koivisto, Y. Levi-Kalisman, R.D. Kornberg, M.
Pettersson and H. Häkkinen, "Electronic and vibrational signatures
of the Au102(pMBA)44 cluster", J. Am. Chem. Soc. 133, 3752 (2011).