EU REACTION project develops diabetes management tools
24 June 2014
The EU funded REACTION project has developed a set of software tools and devices to help both diabetes patients and medical staff better manage the condition.
The REACTION technology platform provides integrated, professional, management and therapy services to diabetes patients in different healthcare regimes across Europe, including:
- professional decision support for in-hospital environments;
- safety monitoring for dosage and compliance;
- long term management of outpatients in clinical schemes;
- care of acute diabetic conditions; and
- support for self management and life-style changes for diabetic patients.
Patients at the Medical University of Graz in Austria and patients at primary care who are being treated at the Chorleywood Health Centre in the UK have been using the system to manage their condition and giving feedback that has helped the researchers to design a system that is effective and intuitive.
In the hospital, nurses and doctors both said that the tools used (REACTION GlucoTab system) helped them to create better care plans for their patients. The improved on-site documentation and predictions provided by the system meant that they could give more accurate doses of insulin. The result is that patients’ glucose levels stayed far more stable in the recommended target range.
In Chorleywood Health Centre, patients used the system to keep a close eye on their symptoms and their vital signs. They could then share these readings with their doctor or nurse, and were much better able to stay healthy as a result.
The project teams developed a number of tools, including:
- GlucoTab, a tablet-PC-based system that advises doctors and nurses on the best course of treatment for each patient. The Gateway supports Continua and other protocols and exports data to different back-ends. It is based on open standards.
- Nutrition App, a smartphone app that allows patients to monitor their nutrition. The app exports data to a back-end server. It stores dietary information and weekly lists of meals and can export into the REACTION database using the service layer.
- Multi-Protocol Home Monitoring Gateway is a software-based gateway running on a standard PC-platform with or without a user interface.
- Chip-based IR Glucose Sensor. The sensor is based on infrared difference absorption spectroscopy on perfusion solution in a disposable chip, attached to a non-disposable, wearable electronics and connected to a microdialysis catheter.
- Wireless Sensor (ePatch) for Heart Rate Monitoring. The ePatch is a miniaturised monitoring system applied to the skin surface with a skin friendly adhesive. The ePatch primarily records ECG but in REACTION the technology has been further developed towards the needs in diabetes treatment. Activity sensors and wireless data communication using ZigBee or Bluetooth have been integrated.
- Patient Portal. The portal is a comprehensive diabetes data management system designed to enable sharing of information between clinicians and patients and supporting the patients in managing their diabetes.
- Clinical Portal. The portal is a software application to manage information collected from sensors, patient portal and care plan in order to provide clinical management of diabetes patients.
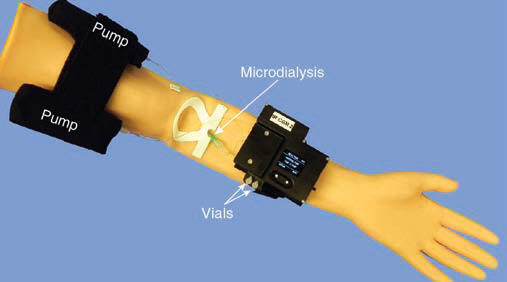
The IR glucose sensor

Zigbee home monitoring platform
The project has also developed a semantic search tool, notification handler, risk models, risk management tool, a glucose-insulin-glucagon model, and a software infrastructure for connectivity and data communications.
The social, economic and legal implications of the technology were studied to better understand what might make tools like this more socially or professionally acceptable.
The REACTION lucoTab system was CE marked and can now be used in a clinical routine. The monitoring devices adapted for primary care patients and the gateway passed safety and EMC tests and have also obtained a CE mark. Finally, in May 2014 the GlucoTab system won the research prize for Human-Technology-Interface in the category “economic applications” awarded by the government of the federal state of Styria in Austria.
Further information
The project's website: www.reaction-project.eu
Link to the project on CORDIS: http://cordis.europa.eu/projects/rcn/108157_en.html
Brochure summarising the project's achievements and products:
www.reactionproject.eu/downloads/marketing/
Reaction_23x22cm_310314_WEB.pdf