OJ Bio develops prototype hand-held disease diagnostics lab
7 November 2014
Newcastle company OJ Bio has successfully completed the prototype development of a portable wireless point-of-care diagnostic system that accurately and quickly identifies the presence of a range of viruses and bacteria in patient fluid samples.
Formal clinical trials using the device to detect a number of respiratory viruses are planned to begin in early 2015. The company has been supported by Innovate UK’s (formerly the Technology Strategy Board) Biomedical Catalyst Fund.
The system combines new test assays carried on a multichannel biochip with a specialist reading device and supporting diagnostic software that can be carried on a mobile app, PC or the device itself.
Importantly, the diagnostic device can be used at a patient’s bed side or other point-of-care location, such as a GP surgery or pharmacy, with the results being available immediately and without samples being sent for laboratory analysis. This capability also enables the device to be used in the patient’s home.


The state-of-the-art biosensors at the heart of the OJ Bio system bring together advanced technology from the company’s two parent organisations: Japan Radio Company (JRC) and Orla Protein Technologies.
JRC has developed Surface Acoustic Wave (SAW) electronic chips which are coated with disease-specific biocapture surfaces developed by Orla — see image below.
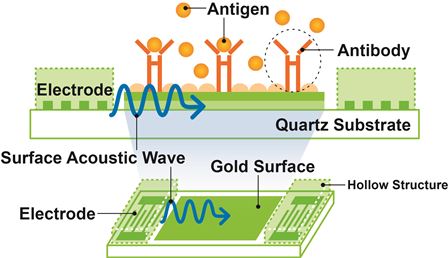
Diagram showing the biocapture process of the
surface acoustic wave chips
When biological samples, from serum, urine, saliva or blood, are applied to the biochip (held in a disposable cartridge), the presence of a disease antigen causes a shift in the phase angle of the surface acoustic wave passing across the chip surface and this is translated into an electronic signal.
This signal can then be detected and its presence (or absence) determined, providing an unequivocal pass or fail result for the particular disease being tested for. Bluetooth connection of the reading device to special diagnostic software enables the test results to be displayed within seconds.
OJ Bio’s Biomedical Catalyst Fund work has so far focussed on research into the detection and diagnosis of the influenza A and B viruses, common flu strains previously linked to some major epidemics, and Respiratory Syncytial Virus (RSV), a major cause of coughs and chest infections.
In addition, a number of other complementary projects are also underway to detect the presence of, for example, periodontal gum disease and HIV.
Dale Athey, chief executive of OJ Bio, said: “Our new device provides a low cost test that dramatically improves the speed of diagnosis and treatment that should hit the disease at source and limit its ability to spread. However, the flexibility of the technology platform has wide ranging diagnostic testing potential, not only for the detection of human medical infections and conditions, but also for use in animal testing by veterinary practices.
“Recent progress has enabled us to accelerate the development of our system and projects are underway with partners in several market sectors which involve the use of the technology in a wide range of medical applications.”
OJ Bio owns the IP for biosurface and SAW technology and is working with clients by assisting with technology support and product development to clinical trial stage. Bio assay development is continuing in the UK and manufacturing facilities are already under development in Japan.
Also home use Bio Assay development continues in the UK and manufacturing facilities are already in development in Japan.